Sodom & Gomorrah Update #2
 The following article was provided by Dr. T. V. Oommen who was one of the members of our October 2000 Noah’s Ark Tour. He had visited the Gomorrah site in December 1998 while on a tour organized by Ron Wyatt. He was able to bring back some ash and sulfur samples from the site at that time (samples graciously collected by Ron for him). A more detailed coverage may be found on his web site at www.biblediscoveries.com
The following article was provided by Dr. T. V. Oommen who was one of the members of our October 2000 Noah’s Ark Tour. He had visited the Gomorrah site in December 1998 while on a tour organized by Ron Wyatt. He was able to bring back some ash and sulfur samples from the site at that time (samples graciously collected by Ron for him). A more detailed coverage may be found on his web site at www.biblediscoveries.com
Sulfur and Ash from Gomorrah
by Dr. T.V. Oommen
“Then the LORD rained down burning sulfur on Sodom and Gomorrah – from the LORD out of the heavens…” (Gen. 19:24)
You have read much about the investigations carried out by Ron Wyatt, Bill Fry and others on the impressive ashen ruins found around the Dead Sea. My own familiarity with these sites is limited to the Gomorrah site (Ron Wyatt’s identification) which is shown on the map.
My contribution to the investigations of the ash and the sulfur samples I had obtained from the site is described below.

Lab Analysis of the Ash
Being a chemist myself, I tested the samples I had brought back to the USA in my lab. This revealed that the sample had two major components: (i)ash, and (ii) elemental sulfur. Other items found occasionally were rocks (with some sulfur deposit), pebbles, and charcoal.
Ash Test
There have been reports which say that the ash is mostly calcium sulfate (gypsum) which is chemically inactive. I added dilute hydrochloric acid to the ash, and it foamed immediately, but no specific smell was noticed.
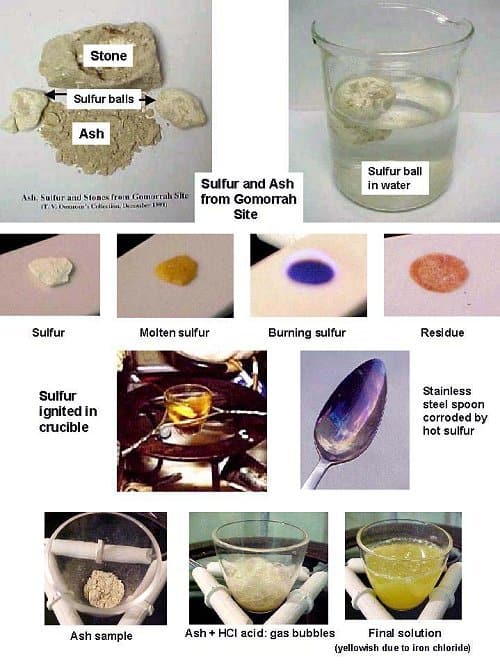
This gives the indication that the ash contains carbonate, and since limestone (calcium carbonate) is found as a main component of rock in many parts of the world including the Gomorrah area, the ash could be mostly limestone; in addition there were some acid insoluble residues which could be silicates and calcium sulfate which I did not try to identify. Apparently the destruction either did not decompose the limestone we see in the ash, or if did, the lime (calcium oxide), recombined with atmospheric carbon dioxide to form the carbonate again. The ash also contained iron compounds (perhaps iron oxide) which gave the solution after treatment with the acid a yellow color (due to ferric chloride).
Sulfur
The presence of sulfur in the ash is unmistakable. It occurs as small granules as well as large chunks, sometimes round, as large as an egg (often encased). These ‘sulfur balls’ are pale yellow, unlike normal sulfur which is clear yellow. Also, these balls are light and are easily crushed into powder when pressed.
 The sulfur ball I tested floated in water, and had a specific gravity of 0.9 (water: 1.0). Common sulfur has a specific gravity of 2.0.
The sulfur ball I tested floated in water, and had a specific gravity of 0.9 (water: 1.0). Common sulfur has a specific gravity of 2.0.
A small piece cut from the sulfur ball was burned with an open flame. As is typical of sulfur, it melted to a dark reddish viscous liquid. Then it caught fire and burned with a bluish flame. The pungent gas, sulfur dioxide (smell of burnt matches) was released. There was a red-orange residue left on the porcelain spatula. This could be red iron oxide either left in the sample or formed by oxidation of some iron containing material left in the sulfur during the heating. Another sample I cut from the interior was almost pure sulfur, and it did not give the reddish residue; only some grayish ash was left.

Another sample was heated in a crucible avoiding flame, and the reddish liquid could be seen more easily. Yet another sample was burned in a stainless steel spoon which discolored and corroded the spoon.

How Were the Sulfur Balls Created?
Sulfur balls of the type I described are very rare. Sulfur deposits found near volcano mouths are in a deposited form. I have visited some regions in Italy with such formations. The ash found in the Sodom & Gomorrah sites is peculiar too. In most places the ash consists of silicate material (I have some Mt. St. Helens ash samples).
What I speculate is the following: Sodom and Gomorrah (and the other three cities) were destroyed by massive ground eruptions in the valley of Siddim (present-day Dead Sea). The Dead Sea area had tar pits according to Gen. 14:10, when it was called the valley of Siddim. Since the Dead Sea itself is believed to have been formed during the catastrophe, it is possible that like Pompeii, the surrounding cities were overwhelmed by the fire and brimstone and ash from the explosions. The actual explosions would be in the region of the Dead Sea which is a deep pit (its northern bank is 1300 ft below sea level, and the bottom of the Sea is another 1300 ft further below at the northern end.
During the ground explosions the valley of Siddim was broken up, and the limestone was shattered and pulverized; these shot up into the sky along with sulfurous gases and steam. The Bible does say that dense smoke like from a furnace was seen by Abraham the next morning (Gen. 19.27-28).
It is important to realize that this catastrophic event was caused by divine wrath; it was not just another natural event. During normal volcanic eruptions there is always the release of steam, and sulfur gases such as hydrogen sulfide (smell of rotten eggs) and sulfur dioxide. These gases can suffocate people.
It is known from chemistry that hydrogen sulfide and sulfur dioxide can react to form elemental sulfur and water. So at the elevated temperatures of the eruption, sulfur was chemically generated and balls of molten sulfur were formed which adsorbed air and some ash particles, and became round as they fell down, probably encased in the ash, eventually solidifying into a porous solid which would be light (for a similar reason, charcoal is lighter than water because of inclusion of air, and is porous).
It was not possible for the sulfur to crystallize because of the air and the ash particles in it. The Genesis statement that sulfur came down from heaven is an observed fact because it literally fell from the sky, though not originating there. Sulfur is rendered “brimstone” (burning stone) in older English Bibles, a true translation from the original tongues.
Anyone who doubts the existence of the Sodom & Gomorrah ruins should pay a visit and just look around and feel the ruins!



